LOCATION
340 East 1st Avenue, Suite 333 Broomfield, CO 80020
PHONE
720-446-8675
BUSINESS HOURS
- Mon - Fri
- -
- Sat - Sun
- Closed
Response Rates
10,000+ Treatments Completed
TMS Vs Fisher Wallace
Fisher Wallace Stimulator® may provide a suitable option if wanting to avoid medications or if medication or therapy has proven ineffective.
Want to know more about TMS? Check out this in-depth guide to TMS therapy with transparent and easy to understand explanations about TMS processes, protocols, and treated conditions.
TMS Vs Fisher Wallace
Fisher Wallace Stimulator® may provide a suitable option if wanting to avoid medications or if medication or therapy has proven ineffective.
Want to know more about TMS? Check out this in-depth guide to TMS therapy with transparent and easy to understand explanations about TMS processes, protocols, and treated conditions.

Fisher Wallace Stimulator® may provide a suitable option if wanting to avoid medications or if medication or therapy has proven ineffective.
TMS Vs Fisher Wallace
Want to know more about TMS? Check out this in-depth guide to TMS therapy with transparent and easy to understand explanations about TMS processes, protocols, and treated conditions.
Can TMS be administered at Home?
TMS as a treatment for depression is growing in popularity due to its effectiveness, safety profile, and tolerability. Furthermore, TMS is being researched and utilized in the treatment of many additional mental health conditions such as OCD, PTSD, anxiety disorders, and other conditions. TMS can be costly, however, particularly if you do not have insurance coverage, and it requires going into a medical office to receive treatment as the device is large and treatments must be administered under the guidance of a certified TMS technician with supervision from a prescribing physician.
Read more: TMS therapy pricing →
To address these barriers to TMS treatment, several medical technology companies have developed therapy somewhat like TMS but using a different type of neurostimulation self-administered via wearable devices at home. This article aims to review one of the most well-known of these products – the Fisher Wallace Stimulator – and to provide a comparison for this vs TMS. A similar product is the
AlphaStim® 100 and other products are likewise available or potentially coming on to the market pending research outcomes.
Fisher Wallace Stimulator
Fisher Wallace labels themselves as 'a patient-owned company’. The initial parent company developed the Fisher Wallace Stimulator® and were given FDA clearance for this device in 1990 for the treatment of depression, anxiety, and insomnia following several successful trials.
The stimulator works on the same principles as TMS using 'neuromodulation.'
"Neuromodulation is technology that acts directly upon nerves. It is the alteration—or modulation—of nerve activity by delivering electrical or pharmaceutical agents directly to a target area." - 17 Jul 2023 https://www.neuromodulation.com
The Fisher Wallace Stimulator® directs low-intensity
electrical waves (or microcurrent) onto superficial areas of the scalp via tACS-Transcranial Alternating Current Stimulation and is referred to more broadly as Cranial Electrotherapy Stimulation (CES). tACS has similarities to TMS and to
ECT-Electrical Convulsive Therapy, but uses a much
milder and superficial electrical current to stimulate brain activity. It does not cause a seizure or require anesthesia (as does ECT) and can be self-administered at home. TMS on the other hand, uses magnetic fields to drive neuronal firing. tACS is not equivalent to tDCS-Transcranial Direct Current Stimulation, which also has studies showing benefit in treating depression and other mental health conditions.
The exact mechanism of action and explanation for why tACS may work in depression, anxiety, and insomnia is not fully understood, but like other neuromodulation strategies, the belief is that repetitive stimulation of neurons at specific locations can lead to larger changes in faulty networks that regulate mood, anxiety, and sleep. There is also research showing that neuromodulation also influences neurotransmitter production (such as serotonin) and can lead to reduction in cortisol levels.
Use of the Fisher Wallace Stimulator® involves once or twice daily 20-minute self-administered sessions for approximately 1 month. The stimulations are delivered via a wearable headband with electrodes placed directly on the temples. There are distinct levels of intensity that can be increased by the user via a handheld device depending on tolerability and response. The procedure is non-invasive, considered to be generally safe, and has been shown in several studies to relieve depression, anxiety, and insomnia symptoms or to improve the efficacy of anti-depressants.
Read more: TMS therapy for Depression→
More rigorous systematic reviews of CES devices have not shown sufficient evidence to support reliable benefit, however. A Cochrane review from 2014 and a VA review from 2018 both concurred there was insufficient evidence to support CES with alternative current as effective for treating depression or other neuropsychiatric conditions, but that there was some possible benefit for treating anxiety with depression. The FDA announced in 2019 that there was no longer sufficient evidence for CES as a treatment for depression and requested new trials be undertaken to support continued use for this indication.
Fisher Wallace reports they have delivered some 100,000 Version 1.0 devices previously and are currently working on completion of a Version 2.0 device, OAK, that they are hoping to regain FDA approval for. The original device was available for purchase ($499 at one time) and could be reimbursed via FSA and HSA funds and sometimes covered by insurance. The device required a physician prescription. The OAK device is currently listed as being ‘sold out’ on the Fisher Wallace website with a cost of $399. The expected date for pilot run manufacturing is listed as Q2 of 2024.
A
recall was initiated in Spring of 2023 for faulty battery installation on previous Fisher Wallace models that could lead to pain, discomfort, irritation, or even burns if not addressed. Customers received notification for corrective action steps from Fisher Wallace to prevent such issues. Rare reports of SAEs (serious adverse events) have been reported by customers using the device and have included reports of stroke, memory loss, atrial fibrillation, bradycardia, and seizures. Whether these events were actually caused by device use, however, is unknown and these incidents have been extremely rare. Less severe side effects have also been reported but are generally mild and short-lived during use of the device.
Fisher Wallace vs TMS-Transcranial Magnetic Stimulation
Transcranial Magnetic Stimulation also treats brain disorders via neuromodulation. However, there are significant differences between TMS and CES devices. For one, TMS targets a specific area of the brain, whereas CES devices do not target a specific area and are more of a 'one size fits all' application.
Second, TMS utilizes magnetic waves to indirectly stimulate neuronal firing in the cortex. This is like a focal MRI in terms of method and intensity.
Read more about the mechanism of action for TMS on our page - How Does TMS Work →
CES devices alternatively utilize a very mild electrical current to stimulate nerves outside the brain.
Both devices can cause mild side effects such as discomfort at the treatment site, headaches, or light-headedness. Seizures are very rare in TMS (1 in 60,000 or more treatments). In general, there are few safety concerns or contraindications with either.
CES devices are utilized at home by the patient, whereas TMS occurs in an office setting under supervision of a physician and TMS technician. A CES device is a one-time purchase, whereas TMS is typically provided for a set number of sessions (most commonly 36). Insurance can cover both treatment modalities for depression, but much more consistently with TMS. Daily sessions are required with both, although TMS is typically M-F only, whereas a CES device can be utilized on weekends as well. A course of treatment with a Fisher Wallace Stimulator® typically entails 2-4 weeks to start and then may continue based on response. TMS typically involves 6-9 weeks of treatment before stopping and then repeating TMS treatment in time based on need.
Read more:
TMS therapy side effects →
Although there are studies indicating benefit from CES for mild to moderate depression, the cumulative evidence is not clear and is less robust than the quantity and quality of data supporting the use of TMS for Treatment Resistant Major Depression. TMS has a strong track record for robust response (meaning 50% or better improvement on a rating scale) and remission (absence of depression based on a similar rating scale).
Inspire TMS Denver tracks all patient outcomes, and we achieve 75-80% response and 40-45% remission rates for the patients we treat.
In conclusion, if mild to moderate depression is present, devices such as the Fisher Wallace Stimulator® may provide a suitable option if wanting to avoid medications or if medication or therapy has proven ineffective. If more severe clinical depression is present, however, there is more limited evidence currently to support the use of such devices. TMS on the other hand, has an excellent track record with support from many governing bodies for treating Major Depressive Disorder. Contact us if wanting to learn more about TMS and to see if this might be a good treatment option for you or a loved one today.


Slide title
Write your caption hereButton
Slide title
Write your caption hereButton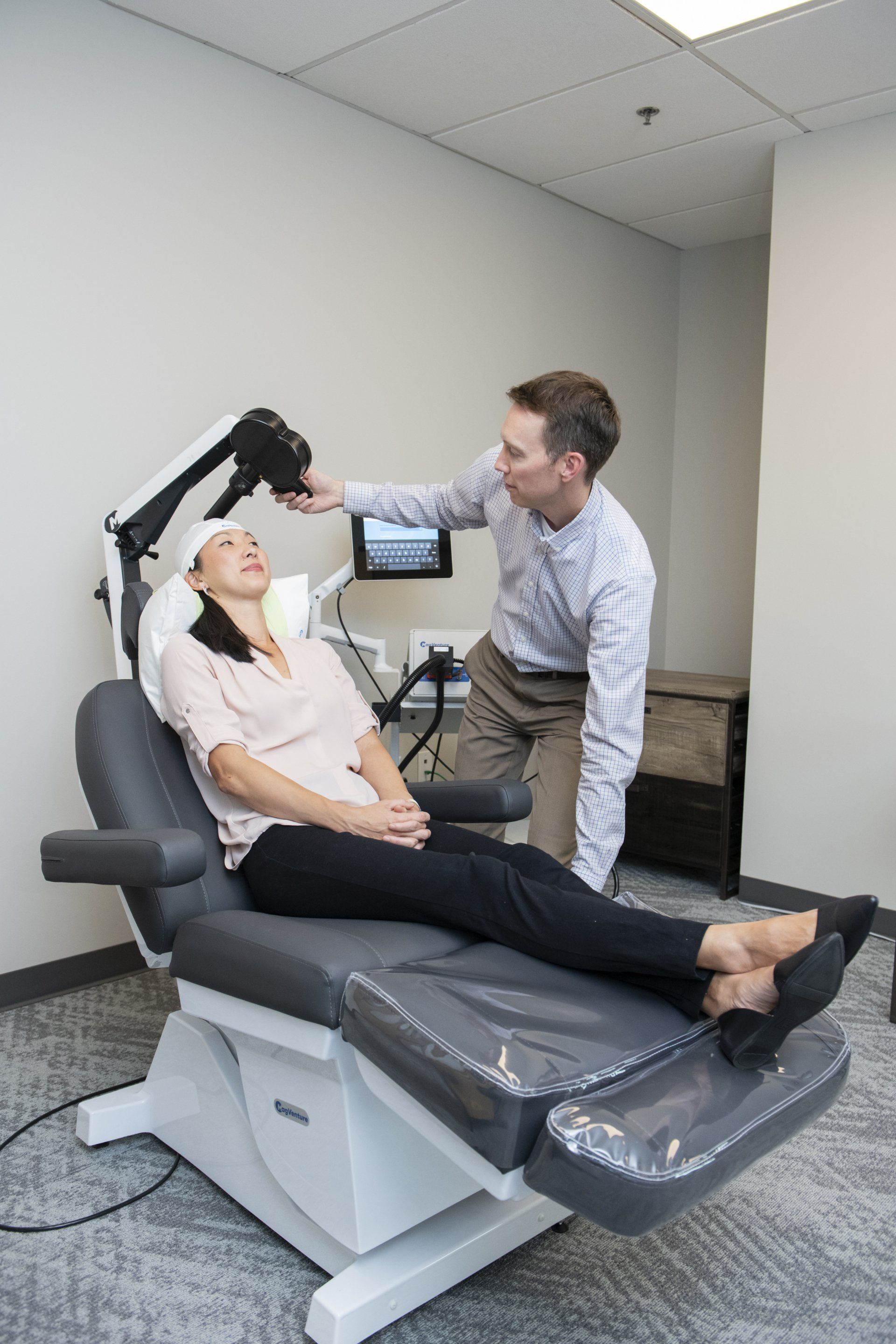
Slide title
Write your caption hereButton
What Makes Us Different
Free TMS Therapy Guides, Quizzes & Phone Consultations

For more visit our video library →
Awards & Accreditations
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
Company Name
the tms journey
A Step By Step Process

Book your free telephone consultation with Dr. Clinch and use this time to ask any questions or voice any concerns about TMS. If there are no contraindications to treatment, you are a good candidate, and you wish to proceed with a full evaluation, we will schedule a full intake. You will be sent an invite to our confidential patient portal and forms for review and completion that expedite care.
Shortly after this, you will be seen in person for the full TMS evaluation. This will provide adequate information for us to then submit prior authorization for TMS coverage to your insurer. If seeking care off-label through self-pay, prior authorization is not needed. We then schedule your first and all subsequent treatment sessions. We obtain prior authorization and inform you of all costs prior to starting care.
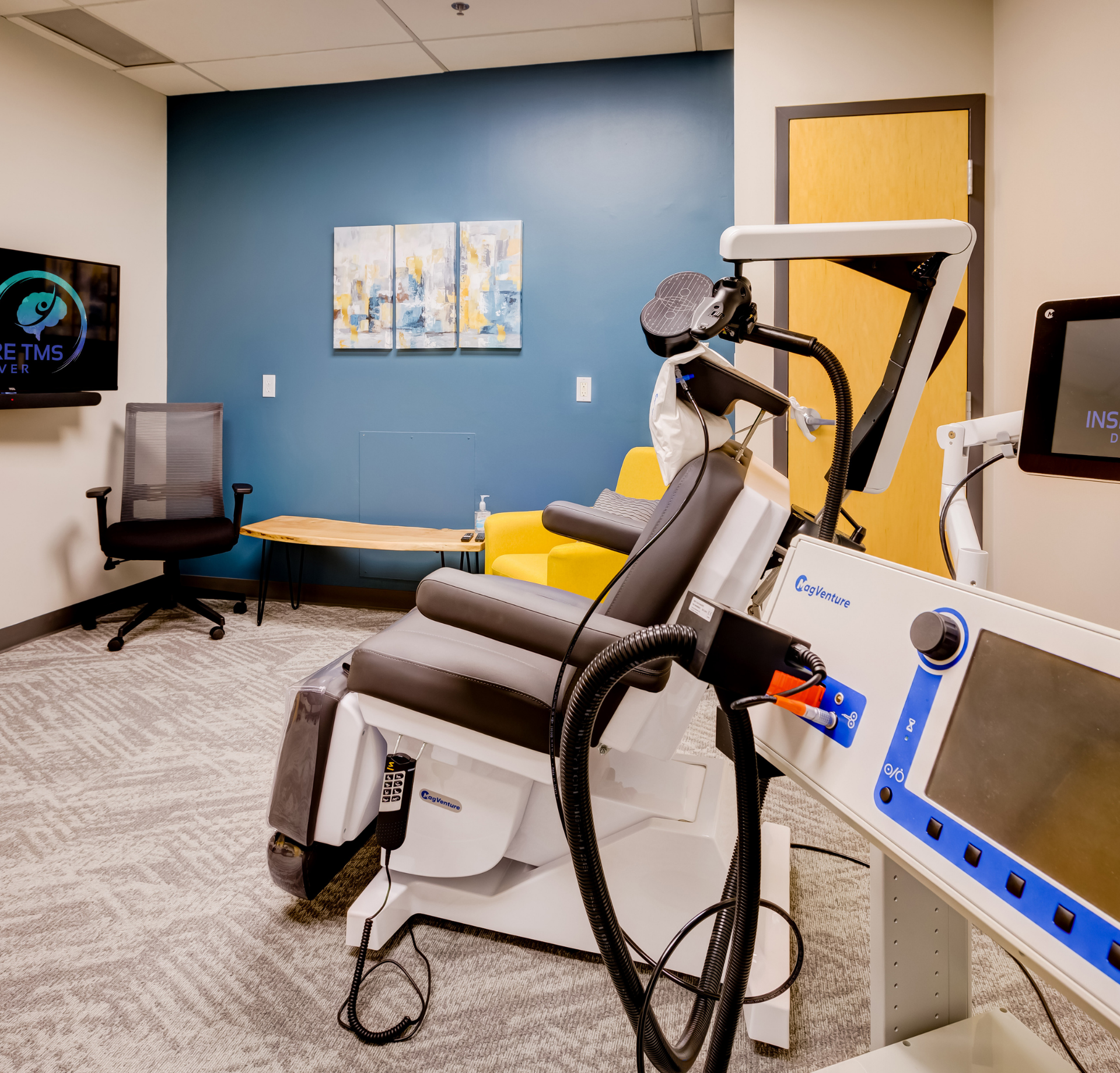
Come in for your first treatment which starts with a 'mapping' to establish your unique treatment intensity and location. Following this and at all subsequent sessions, you will recline in a motorized chair, similar to a dental visit. You can then relax, listen to music, watch TV, read or chat during the treatment. At the end of your sessions, you can drive and return to your day as normal.
Samuel B. Clinch, M.D
Medical Director
Our shared inspiration is to alleviate mental illness and improve the mental wellbeing of the patients we treat. We respect all backgrounds and cultures and want to hear our patient’s stories to best guide care. During treatment, we reinforce positive wellness practices, help maximize lifestyle modifications, and integrate rTMS therapy into a patient’s overall mental and physical health treatment.


Free Consultation
Call 720-446-8675 now, or complete the form below to request a call back.
Contact Us
We will get back to you as soon as possible.
Please try again later.
Contact Us
LOCATION
340 East 1st Avenue, Suite 333
Broomfield, CO 80020
What Happens After I Send My Message?
A member of our team will confirm your free consultation appointment within one business day.
Discover if TMS is right for you and answer all your queries about treatment, eligibility and costs.
Take the First Step Towards Your Mental Well-being Today
Can TMS be administered at Home?
TMS as a treatment for depression is growing in popularity due to its effectiveness, safety profile, and tolerability. Furthermore, TMS is being researched and utilized in the treatment of many additional mental health conditions such as OCD, PTSD, anxiety disorders, and other conditions. TMS can be costly, however, particularly if you do not have insurance coverage, and it requires going into a medical office to receive treatment as the device is large and treatments must be administered under the guidance of a certified TMS technician with supervision from a prescribing physician.
Read more: TMS therapy pricing →
To address these barriers to TMS treatment, several medical technology companies have developed therapy somewhat like TMS but using a different type of neurostimulation self-administered via wearable devices at home. This article aims to review one of the most well-known of these products – the Fisher Wallace Stimulator – and to provide a comparison for this vs TMS. A similar product is the
AlphaStim® 100 and other products are likewise available or potentially coming on to the market pending research outcomes.
Fisher Wallace Stimulator
Fisher Wallace labels themselves as 'a patient-owned company’. The initial parent company developed the Fisher Wallace Stimulator® and were given FDA clearance for this device in 1990 for the treatment of depression, anxiety, and insomnia following several successful trials.
The stimulator works on the same principles as TMS using 'neuromodulation.'
"Neuromodulation is technology that acts directly upon nerves. It is the alteration—or modulation—of nerve activity by delivering electrical or pharmaceutical agents directly to a target area." - 17 Jul 2023 https://www.neuromodulation.com
The Fisher Wallace Stimulator® directs low-intensity
electrical waves (or microcurrent) onto superficial areas of the scalp via tACS-Transcranial Alternating Current Stimulation and is referred to more broadly as Cranial Electrotherapy Stimulation (CES). tACS has similarities to TMS and to
ECT-Electrical Convulsive Therapy, but uses a much
milder and superficial electrical current to stimulate brain activity. It does not cause a seizure or require anesthesia (as does ECT) and can be self-administered at home. TMS on the other hand, uses magnetic fields to drive neuronal firing. tACS is not equivalent to tDCS-Transcranial Direct Current Stimulation, which also has studies showing benefit in treating depression and other mental health conditions.
The exact mechanism of action and explanation for why tACS may work in depression, anxiety, and insomnia is not fully understood, but like other neuromodulation strategies, the belief is that repetitive stimulation of neurons at specific locations can lead to larger changes in faulty networks that regulate mood, anxiety, and sleep. There is also research showing that neuromodulation also influences neurotransmitter production (such as serotonin) and can lead to reduction in cortisol levels.
Use of the Fisher Wallace Stimulator® involves once or twice daily 20-minute self-administered sessions for approximately 1 month. The stimulations are delivered via a wearable headband with electrodes placed directly on the temples. There are distinct levels of intensity that can be increased by the user via a handheld device depending on tolerability and response. The procedure is non-invasive, considered to be generally safe, and has been shown in several studies to relieve depression, anxiety, and insomnia symptoms or to improve the efficacy of anti-depressants.
Read more: TMS therapy for Depression→
More rigorous systematic reviews of CES devices have not shown sufficient evidence to support reliable benefit, however. A Cochrane review from 2014 and a VA review from 2018 both concurred there was insufficient evidence to support CES with alternative current as effective for treating depression or other neuropsychiatric conditions, but that there was some possible benefit for treating anxiety with depression. The FDA announced in 2019 that there was no longer sufficient evidence for CES as a treatment for depression and requested new trials be undertaken to support continued use for this indication.
Fisher Wallace reports they have delivered some 100,000 Version 1.0 devices previously and are currently working on completion of a Version 2.0 device, OAK, that they are hoping to regain FDA approval for. The original device was available for purchase ($499 at one time) and could be reimbursed via FSA and HSA funds and sometimes covered by insurance. The device required a physician prescription. The OAK device is currently listed as being ‘sold out’ on the Fisher Wallace website with a cost of $399. The expected date for pilot run manufacturing is listed as Q2 of 2024.
A
recall was initiated in Spring of 2023 for faulty battery installation on previous Fisher Wallace models that could lead to pain, discomfort, irritation, or even burns if not addressed. Customers received notification for corrective action steps from Fisher Wallace to prevent such issues. Rare reports of SAEs (serious adverse events) have been reported by customers using the device and have included reports of stroke, memory loss, atrial fibrillation, bradycardia, and seizures. Whether these events were actually caused by device use, however, is unknown and these incidents have been extremely rare. Less severe side effects have also been reported but are generally mild and short-lived during use of the device.
Fisher Wallace vs TMS-Transcranial Magnetic Stimulation
Transcranial Magnetic Stimulation also treats brain disorders via neuromodulation. However, there are significant differences between TMS and CES devices. For one, TMS targets a specific area of the brain, whereas CES devices do not target a specific area and are more of a 'one size fits all' application.
Second, TMS utilizes magnetic waves to indirectly stimulate neuronal firing in the cortex. This is like a focal MRI in terms of method and intensity. Read more about the mechanism of action for TMS on our page - How Does TMS Work →
CES devices alternatively utilize a very mild electrical current to stimulate nerves outside the brain.
Both devices can cause mild side effects such as discomfort at the treatment site, headaches, or light-headedness. Seizures are very rare in TMS (1 in 60,000 or more treatments). In general, there are few safety concerns or contraindications with either.
CES devices are utilized at home by the patient, whereas TMS occurs in an office setting under supervision of a physician and TMS technician. A CES device is a one-time purchase, whereas TMS is typically provided for a set number of sessions (most commonly 36). Insurance can cover both treatment modalities for depression, but much more consistently with TMS. Daily sessions are required with both, although TMS is typically M-F only, whereas a CES device can be utilized on weekends as well. A course of treatment with a Fisher Wallace Stimulator® typically entails 2-4 weeks to start and then may continue based on response. TMS typically involves 6-9 weeks of treatment before stopping and then repeating TMS treatment in time based on need.
Read more:
TMS therapy side effects →
Although there are studies indicating benefit from CES for mild to moderate depression, the cumulative evidence is not clear and is less robust than the quantity and quality of data supporting the use of TMS for Treatment Resistant Major Depression. TMS has a strong track record for robust response (meaning 50% or better improvement on a rating scale) and remission (absence of depression based on a similar rating scale).
Inspire TMS Denver tracks all patient outcomes, and we achieve 75-80% response and 40-45% remission rates for the patients we treat.
In conclusion, if mild to moderate depression is present, devices such as the Fisher Wallace Stimulator® may provide a suitable option if wanting to avoid medications or if medication or therapy has proven ineffective. If more severe clinical depression is present, however, there is more limited evidence currently to support the use of such devices. TMS on the other hand, has an excellent track record with support from many governing bodies for treating Major Depressive Disorder. Contact us if wanting to learn more about TMS and to see if this might be a good treatment option for you or a loved one today.


Slide title
Write your caption hereButton
Slide title
Write your caption hereButton
Slide title
Write your caption hereButton
What Makes Us Different

As Seen On Colorado's Best

For more visit our video library →
Awards & Accreditations
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
Company Name
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
Company Name
the tms journey
A Step By Step Process
Book your free telephone consultation with Dr. Clinch and use this time to ask any questions or voice any concerns about TMS. If there are no contraindications to treatment, you are a good candidate, and you wish to proceed with a full evaluation, we will schedule a full intake. You will be sent an invite to our confidential patient portal and forms for review and completion that expedite care.
Shortly after this, you will be seen in person for the full TMS evaluation. This will provide adequate information for us to then submit prior authorization for TMS coverage to your insurer. If seeking care off-label through self-pay, prior authorization is not needed. We then schedule your first and all subsequent treatment sessions. We obtain prior authorization and inform you of all costs prior to starting care.
Come in for your first treatment which starts with a 'mapping' to establish your unique treatment intensity and location. Following this and at all subsequent sessions, you will recline in a motorized chair, similar to a dental visit. You can then relax, listen to music, watch TV, read or chat during the treatment. At the end of your sessions, you can drive and return to your day as normal.
Our shared inspiration is to alleviate mental illness and improve the mental wellbeing of the patients we treat. We respect all backgrounds and cultures and want to hear our patient’s stories to best guide care. During treatment, we reinforce positive wellness practices, help maximize lifestyle modifications, and integrate rTMS therapy into a patient’s overall mental and physical health treatment.
Samuel B. Clinch, M.D
Medical Director
Free Consultation
Call 720-446-8675 now, or complete the form below to request a call back.
Contact Us
We will get back to you as soon as possible.
Please try again later.
Contact Us
LOCATION
340 East 1st Avenue, Suite 333
Broomfield, CO 80020
What Happens After I Send My Message?
A member of our team will confirm your free consultation appointment within one business day.
Discover if TMS is right for you and answer all your queries about treatment, eligibility and costs.
Take the First Step Towards Your Mental Well-being Today
Can TMS be administered at Home?
TMS as a treatment for depression is growing in popularity due to its effectiveness, safety profile, and tolerability. Furthermore, TMS is being researched and utilized in the treatment of many additional mental health conditions such as OCD, PTSD, anxiety disorders, and other conditions. TMS can be costly, however, particularly if you do not have insurance coverage, and it requires going into a medical office to receive treatment as the device is large and treatments must be administered under the guidance of a certified TMS technician with supervision from a prescribing physician.
Read more: TMS therapy pricing →
To address these barriers to TMS treatment, several medical technology companies have developed therapy somewhat like TMS but using a different type of neurostimulation self-administered via wearable devices at home. This article aims to review one of the most well-known of these products – the Fisher Wallace Stimulator – and to provide a comparison for this vs TMS. A similar product is the
AlphaStim® 100 and other products are likewise available or potentially coming on to the market pending research outcomes.
Fisher Wallace Stimulator
Fisher Wallace labels themselves as 'a patient-owned company’. The initial parent company developed the Fisher Wallace Stimulator® and were given FDA clearance for this device in 1990 for the treatment of depression, anxiety, and insomnia following several successful trials.
The stimulator works on the same principles as TMS using 'neuromodulation.'
"Neuromodulation is technology that acts directly upon nerves. It is the alteration—or modulation—of nerve activity by delivering electrical or pharmaceutical agents directly to a target area." - 17 Jul 2023 https://www.neuromodulation.com
The Fisher Wallace Stimulator® directs low-intensity
electrical waves (or microcurrent) onto superficial areas of the scalp via tACS-Transcranial Alternating Current Stimulation and is referred to more broadly as Cranial Electrotherapy Stimulation (CES). tACS has similarities to TMS and to
ECT-Electrical Convulsive Therapy, but uses a much
milder and superficial electrical current to stimulate brain activity. It does not cause a seizure or require anesthesia (as does ECT) and can be self-administered at home. TMS on the other hand, uses magnetic fields to drive neuronal firing. tACS is not equivalent to tDCS-Transcranial Direct Current Stimulation, which also has studies showing benefit in treating depression and other mental health conditions.
The exact mechanism of action and explanation for why tACS may work in depression, anxiety, and insomnia is not fully understood, but like other neuromodulation strategies, the belief is that repetitive stimulation of neurons at specific locations can lead to larger changes in faulty networks that regulate mood, anxiety, and sleep. There is also research showing that neuromodulation also influences neurotransmitter production (such as serotonin) and can lead to reduction in cortisol levels.
Use of the Fisher Wallace Stimulator® involves once or twice daily 20-minute self-administered sessions for approximately 1 month. The stimulations are delivered via a wearable headband with electrodes placed directly on the temples. There are distinct levels of intensity that can be increased by the user via a handheld device depending on tolerability and response. The procedure is non-invasive, considered to be generally safe, and has been shown in several studies to relieve depression, anxiety, and insomnia symptoms or to improve the efficacy of anti-depressants.
Read more: TMS therapy for Depression→
More rigorous systematic reviews of CES devices have not shown sufficient evidence to support reliable benefit, however. A Cochrane review from 2014 and a VA review from 2018 both concurred there was insufficient evidence to support CES with alternative current as effective for treating depression or other neuropsychiatric conditions, but that there was some possible benefit for treating anxiety with depression. The FDA announced in 2019 that there was no longer sufficient evidence for CES as a treatment for depression and requested new trials be undertaken to support continued use for this indication.
Fisher Wallace reports they have delivered some 100,000 Version 1.0 devices previously and are currently working on completion of a Version 2.0 device, OAK, that they are hoping to regain FDA approval for. The original device was available for purchase ($499 at one time) and could be reimbursed via FSA and HSA funds and sometimes covered by insurance. The device required a physician prescription. The OAK device is currently listed as being ‘sold out’ on the Fisher Wallace website with a cost of $399. The expected date for pilot run manufacturing is listed as Q2 of 2024.
A
recall was initiated in Spring of 2023 for faulty battery installation on previous Fisher Wallace models that could lead to pain, discomfort, irritation, or even burns if not addressed. Customers received notification for corrective action steps from Fisher Wallace to prevent such issues. Rare reports of SAEs (serious adverse events) have been reported by customers using the device and have included reports of stroke, memory loss, atrial fibrillation, bradycardia, and seizures. Whether these events were actually caused by device use, however, is unknown and these incidents have been extremely rare. Less severe side effects have also been reported but are generally mild and short-lived during use of the device.
Fisher Wallace vs TMS-Transcranial Magnetic Stimulation
Transcranial Magnetic Stimulation also treats brain disorders via neuromodulation. However, there are significant differences between TMS and CES devices. For one, TMS targets a specific area of the brain, whereas CES devices do not target a specific area and are more of a 'one size fits all' application.
Second, TMS utilizes magnetic waves to indirectly stimulate neuronal firing in the cortex. This is like a focal MRI in terms of method and intensity. Read more about the mechanism of action for TMS on our page - How Does TMS Work →
CES devices alternatively utilize a very mild electrical current to stimulate nerves outside the brain.
Both devices can cause mild side effects such as discomfort at the treatment site, headaches, or light-headedness. Seizures are very rare in TMS (1 in 60,000 or more treatments). In general, there are few safety concerns or contraindications with either.
CES devices are utilized at home by the patient, whereas TMS occurs in an office setting under supervision of a physician and TMS technician. A CES device is a one-time purchase, whereas TMS is typically provided for a set number of sessions (most commonly 36). Insurance can cover both treatment modalities for depression, but much more consistently with TMS. Daily sessions are required with both, although TMS is typically M-F only, whereas a CES device can be utilized on weekends as well. A course of treatment with a Fisher Wallace Stimulator® typically entails 2-4 weeks to start and then may continue based on response. TMS typically involves 6-9 weeks of treatment before stopping and then repeating TMS treatment in time based on need.
Read more:
TMS therapy side effects →
Although there are studies indicating benefit from CES for mild to moderate depression, the cumulative evidence is not clear and is less robust than the quantity and quality of data supporting the use of TMS for Treatment Resistant Major Depression. TMS has a strong track record for robust response (meaning 50% or better improvement on a rating scale) and remission (absence of depression based on a similar rating scale).
Inspire TMS Denver tracks all patient outcomes, and we achieve 75-80% response and 40-45% remission rates for the patients we treat.
In conclusion, if mild to moderate depression is present, devices such as the Fisher Wallace Stimulator® may provide a suitable option if wanting to avoid medications or if medication or therapy has proven ineffective. If more severe clinical depression is present, however, there is more limited evidence currently to support the use of such devices. TMS on the other hand, has an excellent track record with support from many governing bodies for treating Major Depressive Disorder. Contact us if wanting to learn more about TMS and to see if this might be a good treatment option for you or a loved one today.
Featured Reviews

-

“TMS really helped me with depression when it seemed like nothing else did. I had previously tried several different medications and ketamine infusions without seeing much improvement, but TMS has made a massive difference in my mood and quality of life.”
- Andre D.
MashIt 
"Every day I am so exceptionally surprised by how much better I feel since doing the TMS series with Inspire TMS in Broomfield. I completed the series 2 months ago. I am also accomplishing much more now with ease."
FabuFit
“Dr. Clinch, Sydney and Michelle, have been amazing since the beginning. Dr. Clinch took his time to explain everything about TMS on my consultation and was present for all treatment appointments even if it was just to do a quick check-in.”
YesSuits
Too good to be true? Compare the many ups and the few downs of TMS.
Measuring TMS against ECT is like comparing apples to oranges, some similarities but major differences.
Patients have to try multiple medications due to lack of response or side effects.
Free Consultation
What Happens After I Send My Message?
- A member of our team will confirm your free consultation appointment within one business day.
- Discover if TMS is right for you and answer all your queries about treatment, eligibility and costs.
Contact Us
We will get back to you as soon as possible.
Please try again later.
Is TMS Right For You?
We believe rTMS is an underutilized treatment approach. It is safe, non invasive, free of systemic side effects and well tolerated. Discover if TMS is right for you by taking the quiz or booking a consultation.


Slide title
Write your caption hereButton
Slide title
Write your caption hereButton
Slide title
Write your caption hereButton
What Makes Us Different

As Seen On Colorado's Best

For more visit our video library →
Awards & Accreditations
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
Company Name
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
This is a paragraph. Writing in paragraphs lets visitors find what they are looking for quickly and easily.
Company Name
the tms journey
A Step By Step Process
Book your free telephone consultation with Dr. Clinch and use this time to ask any questions or voice any concerns about TMS. If there are no contraindications to treatment, you are a good candidate, and you wish to proceed with a full evaluation, we will schedule a full intake. You will be sent an invite to our confidential patient portal and forms for review and completion that expedite care.
Shortly after this, you will be seen in person for the full TMS evaluation. This will provide adequate information for us to then submit prior authorization for TMS coverage to your insurer. If seeking care off-label through self-pay, prior authorization is not needed. We then schedule your first and all subsequent treatment sessions. We obtain prior authorization and inform you of all costs prior to starting care.
Come in for your first treatment which starts with a 'mapping' to establish your unique treatment intensity and location. Following this and at all subsequent sessions, you will recline in a motorized chair, similar to a dental visit. You can then relax, listen to music, watch TV, read or chat during the treatment. At the end of your sessions, you can drive and return to your day as normal.
Our shared inspiration is to alleviate mental illness and improve the mental wellbeing of the patients we treat. We respect all backgrounds and cultures and want to hear our patient’s stories to best guide care. During treatment, we reinforce positive wellness practices, help maximize lifestyle modifications, and integrate rTMS therapy into a patient’s overall mental and physical health treatment.
Samuel B. Clinch, M.D
Medical Director
Free Consultation
Call 720-446-8675 now, or complete the form below to request a call back.
Contact Us
We will get back to you as soon as possible.
Please try again later.
Contact Us
MAIN OFFICE
720-446-8675
LOCATION
340 East 1st Avenue, Suite 333
Broomfield, CO 80020
What Happens After I Send My Message?
A member of our team will confirm your free consultation appointment within one business day.
Discover if TMS is right for you and answer all your queries about treatment, eligibility and costs.
Take the First Step Towards Your Mental Wellbeing Today
Our Services
Learning Hub
Quick Links
Our Services
Learning Hub
Quick Links
Committed
to Your Individual
Care & Recovery
Our Services
Learning Hub
Quick Links
Business Hours
- Monday
- -
- Tuesday
- -
- Wednesday
- -
- Thursday
- -
- Friday
- -
- Saturday
- Closed
- Sunday
- Closed
Find Us
LOCATION
PHONE & FAX
720-446-8675 | 720-798-6969
All Rights Reserved | Inspire TMS Denver
Information on this site is for reference purposes only. It is not intended to be nor should it be taken as medical advice. Individuals should see a medical professional regarding their symptoms.
Website by Leo Cook Digital Marketing
Committed
to Your Individual
Care & Recovery
Business Hours
- Monday
- -
- Tuesday
- -
- Wednesday
- -
- Thursday
- -
- Friday
- -
- Saturday
- Closed
- Sunday
- Closed
Find Us
LOCATION
PHONE
720-446-8675 | 720-798-6969
Information on this site is for reference purposes only. It is not intended to be nor should it be taken as medical advice. Individuals should see a medical professional regarding their symptoms.
All Rights Reserved | Inspire TMS Denver
Website by Leo Cook Marketing
Business Hours
- Monday
- -
- Tuesday
- -
- Wednesday
- -
- Thursday
- -
- Friday
- -
- Saturday
- Closed
- Sunday
- Closed
Find Us
LOCATION
PHONE & FAX
720-446-8675 | 720-798-6969
National Suicide Prevention Lifeline


Emergencies Call 911
Receive help 24 hours a day
Information on this site is for reference purposes only. It is not intended to be nor should it be taken as medical advice. Individuals should see a medical professional regarding their symptoms.
All Rights Reserved | Inspire TMS Denver
Website by Leo Cook Digital Marketing